Table of Contents
Islet transplantation has emerged as a promising approach for treating Type 1 diabetes. Researchers at Weill Cornell Medicine have developed an innovative technique that integrates Islets of Langerhans with blood vessel cells, offering new hope for patients.
In a preliminary study conducted by researchers at Weill Cornell Medicine, it was shown that the addition of genetically modified human cells to form blood vessels significantly enhances islet transplantation. This integration improved the survival of insulin-producing cells and even contributed to reversing the course of diabetes in the study.
This new approach, which is still under development and testing, opens broad prospects for the use of islet transplantation as a treatment for diabetes. However, it must be emphasized that further research and clinical trials are necessary to ensure the safety and efficacy of this technique before widespread application.
The Islets of Langerhans, located in the pancreas, are clusters of cells that secrete insulin, intricately networked with a specialized network of small blood vessels.
Islet Transplantation Breakthrough
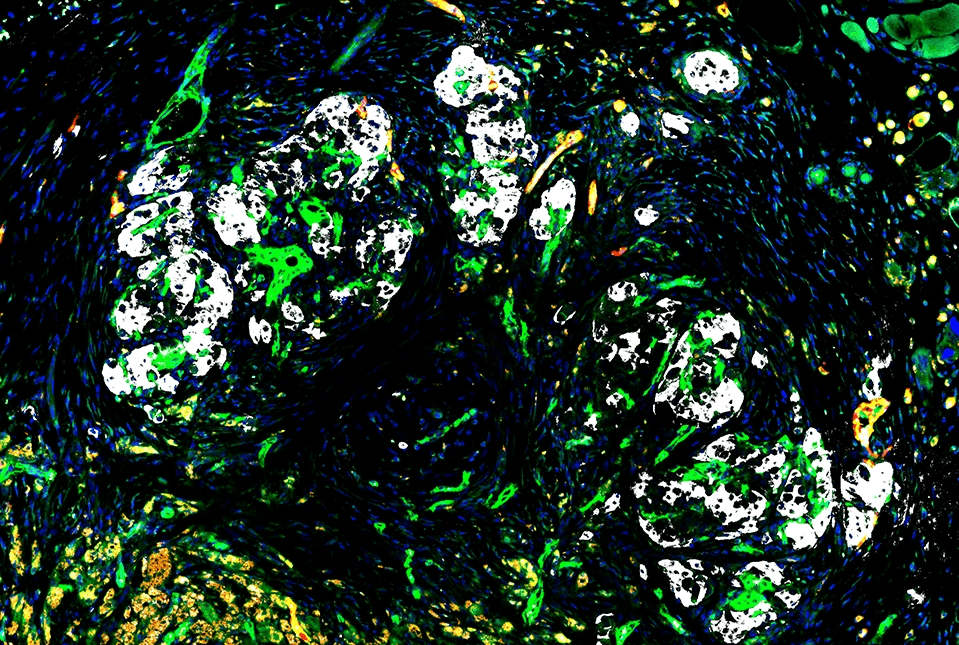
In type 1 diabetes, which affects approximately nine million people worldwide, insulin cells are destroyed by the body’s immune system. Although islet transplantation is a promising treatment option, the only method approved by the U.S. Food and Drug Administration to date faces significant challenges.
In a study published on January 29 in the journal Science Advances, researchers showed that the blood vessel-forming cells they developed, called “reprogrammed vascular endothelial cells” (R-VECs), can overcome some of these challenges by providing robust support to the islets, allowing them to survive and reverse diabetes long-term when transplanted under the skin of mice.
Dr. Ji Lei, a researcher involved in the study, stated, “This work lays the foundation for subcutaneous islet transplantation as a relatively safe and durable treatment option for type 1 diabetes.” However, it must be emphasized that these results are preliminary and require further validation.
Traditional Method Challenges and Precautions
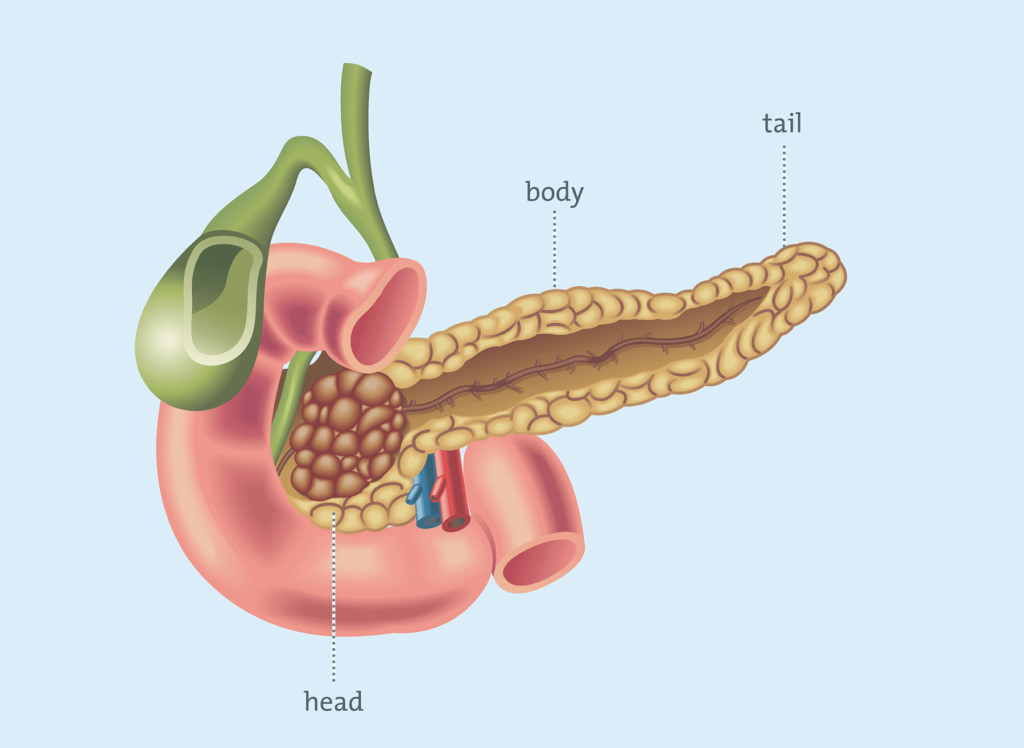
The current approved method for islet transplantation involves injecting the islets into a vein in the liver. This surgical procedure requires long-term use of immunosuppressant drugs to prevent islet rejection, involves relatively uncontrolled islet dispersal, and often becomes ineffective within a few years, likely due in part to a lack of proper supporting cells.
Researchers aspire to develop a method that allows islets to be transplanted to a more controlled and accessible location, such as under the skin, and allows the transplanted tissue to survive longer. They also hope to overcome the issue of immune rejection by using islets and endothelial cells derived from patients’ own cells or engineered to be invisible to the immune system. However, it must be emphasized that comprehensive studies are necessary to evaluate the potential risks and benefits of these technologies before human application.
In the new study, researchers demonstrated the feasibility of long-term subcutaneous islet transplantation using R-VECs as essential support cells.
Dr. Shahin Rafii, a researcher involved in the study, explained, “We have shown that human blood vessel cells transplanted into the subcutaneous tissue of immunocompromised mice immediately connect to the host’s blood circulation, providing immediate nutrition and oxygen, thus enhancing the survival and function of vulnerable islets.” However, it must be emphasized that these results are still in their early stages and require further research to confirm.
Dr. David Redmond added, “Surprisingly, we found that R-VECs adapt when transplanted with islets, supporting the islets with a rich network of new vessels.” However, it must be emphasized that long-term studies are necessary to assess the safety and efficacy of this technique in the long term.
A significant majority of diabetic mice that received islet cells with R-VECs regained normal body weight and showed normal blood sugar control even after 20 weeks, a period that indicates permanent islet cell engraftment in the mouse model of diabetes. Mice that received islet cells but without R-VECs were less successful. However, it must be emphasized that these results are preliminary and require further validation before human application.











Leave a Comment